Silica... More or less, It will mess..!!
#membraneautopsy
Extensive studies of membrane autopsies shows the physical damage of membrane such as increasing salt passage, change in flux and so on . After studying innumerable membranes we found silica plays vital role in most of the physical and irreversible damage of the membrane among the crystallized minerals.
In such cases, the foulant composition contain silica near about 10% - 50% . Aluminum and iron in association with silica accelerate the phenomenon.
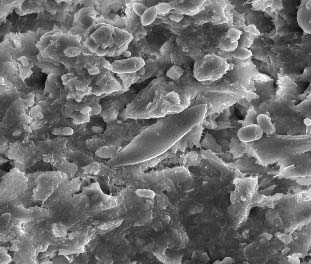
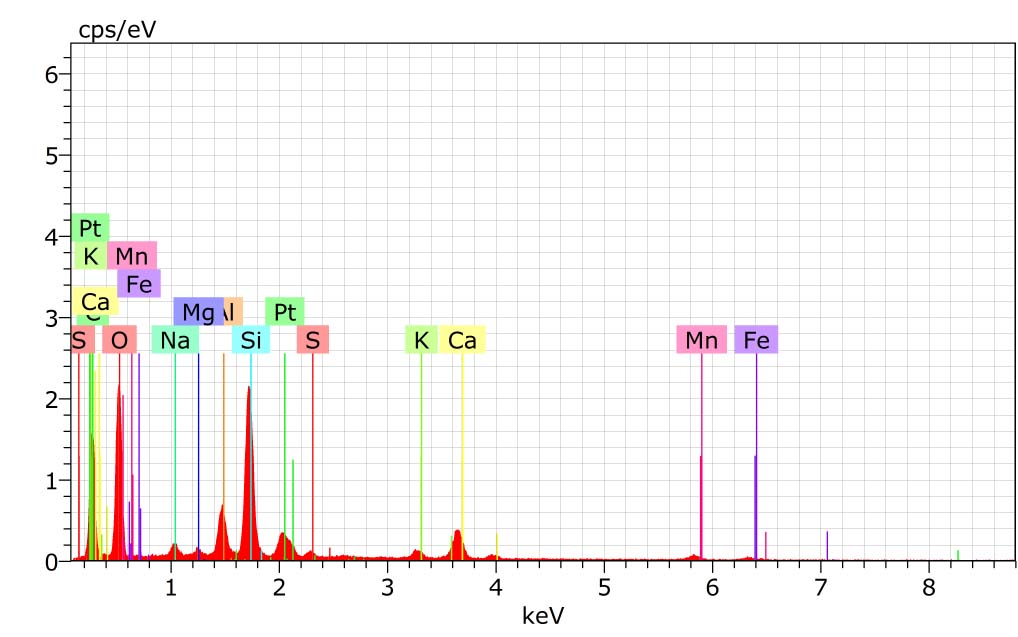
Silica is one of the major culprit which can cause the irreversible damage on membrane surface. It may affect the membrane surface by different ways such as by forming deposition/fouling on membrane or by causing impact on the flux and increasing the trans membrane pressure on membrane etc.
The presence of reactive and colloidal silica in feed water plays an important role in pretreatment of water. It has been observed that while designing system, colloidal silica is rarely analysed. Most of the time, the parameter analysed is reactive silica but not the colloidal silica. It is not necessary that colloidal silica may have similar trend like calcium, magnesium and other minerals and metals. The presence of colloidal silica in water depends on various factors like temperature, pH etc. Simultaneously, this factor affect the solubility of reactive silica. But. unfortunately it is very difficult to obtain trend of Colloidal Silica in water. Though the sample has been drawn in similar conditions and period, we can observe variability in colloidal silica content. Thus, the variation and deviation of silica forms takes place not only as per the pH and temperature but also solubility of silica, saturation level of silica, content of different minerals and metals in water and source of water.
The presence of colloidal silica is commonly found in river water, sea water, followed by pond water and well water. In these circumstances the pretreatment of feed water plays an important role. Apart from raw water clarification followed by ultrafiltration also plays major role in reducing the colloidal silica. The ultrafiltration process removes the colloidal particles higher than the 0.01μm but the size of colloidal silica particles can be smaller up to 0.001μm. So here we understand the limitation of UF filter. Uncertain trend of colloidal silica and limitation of UF make pretreatment more crucial. Hence, to get rid of colloidal silica in feed water good design and healthy operation practices of pretreatment is essential.
In case of the reverse osmosis processes while the separation of salts, some salts may react with silica which enhance the accumulation of other forms of minerals and silica on membrane surface and causes the silica fouling formation. This silica causes impact on the flux and increase the trans membrane pressure. Thus, higher concentration of silica along with changes in pH may increase fouling possibilities on the membrane surface.
The presence of silica foulant leads to panic and harsh cleaning which are responsible for other different types of physical damages. The acid and alkali cleaning causes impact on silica. The possibility of metal silica fouling increases due to frequent acidification of feed water. As pH increases above neutral, silicic acid dissociates into the silicate anion (SiO32-)n. This can react with calcium, magnesium, iron, manganese or aluminum to form insoluble silicates. Among all these aluminium is one of the most powerful element which causes precipitation of silica. The presence of both Al3+ and Fe3+ in the pretreated feed water causes the precipitation of silica even at below the saturation concentration level of silica . Hence, to maintain the concentration of both Al3+ and Fe3+ in feed water it is very important as both Al3+ and Fe3+ salts are used as coagulant in water treatment processes. So, post coagulation concentration of Al3+ and Fe3+ should be below 0.05mg/l or less.
Silica forms a complex with hydrated form of calcium, aluminium, magnesium, and iron elements. This complex form polymerizes and creates colloids. In presence of calcium carbonate and calcium sulphate, amorphous deposits of silica are formed. The colloid forms of silica forms the bridge between organic and inorganic matter, a gel like layer on membrane surface.
Apart from above, silica also reacts with organic components including different types of proteins, humic acids and polysaccharides. The presence of silica along with organic compound in feed water causes comparatively more impact on flux as compared to individual organic or inorganic fouling. The deposition of silica on membrane causes formation of thin film on membrane which also causes impact on polyamide layer. The hydrolysis of trimesoyl chloride (which is the part of polyamide membrane) causes the formation of carboxyl group . The silica may form a bond with carbon in the carboxyl group by substituting the oxygen. The decrease in carboxyl group may cause impact on flux. These changes in carboxyl group causes irreversible damage.
To get the rid of reactive and colloidal silica problem understanding the effective pretreatment is essential and to design effective pretreatment, adequate data of reactive and colloidal silica is required.
So, negligence to the reactive silica and colloidal silica will affect CapEx and OpEx.